Graduate Course: Quantum Physics
- Bohr Atommodell
- Bohr Atom Energy Levels
- What Did Bohr Say About Atoms
- Bohr Atom
- Bohr Atom
- Bohr Atomic Models Worksheet Answers
The Bohr model of the atom, a radical departure from earlier, classical descriptions, was the first that incorporated quantum theory and was the predecessor of wholly quantum-mechanical models. The Bohr model and all of its successors describe the properties of atomic electrons in terms of a set of allowed (possible) values. Atom - Atom - Bohr’s shell model: In 1913 Bohr proposed his quantized shell model of the atom (see Bohr atomic model) to explain how electrons can have stable orbits around the nucleus. The motion of the electrons in the Rutherford model was unstable because, according to classical mechanics and electromagnetic theory, any charged particle moving on a curved path emits electromagnetic.
and John Venables, Dept of Physics and Astronomy, Arizona State University, Tempe, Arizona
The BOHR ATOM
'Anyone who is not shocked by quantum theory has not understood it'
- Neils Bohr
In 1913 Neils Bohr proposed his model of atom which superceded Rutherford's atomic model. Though the planetary model proposed by Rutherford was widely accepted, it fell short on many counts. The nuclear atom proposed by Rutherford was unstable. According to classical theories this atom should collapse. It also failed to explain the discrete spectral lines of elements. Bohr's model of atom could successfully explain the stability of atom by introducing Quantization. It could also explain the Hydrogen spectra. Bohr obtained the value of radius of hydrogen atom and its energy, both of which agree well with experimental results. Was this a coincidence!? Bohr's atomic theory formed the basis for the old Quantum theory. This page concentrates on illustrating the ingredients of the Bohr model.
Bohr's Postulates
(a) The electron revolves in circular orbits around the nucleus which are restricted by the quantization of angular momentum i.e. they revolve in orbits where the angular momentum of electron is an integral multiple of h/2π, where h is Planck's constant.
In these orbits of special radius electron does not radiate energy as expected from Maxwell's laws. These orbits are called stationary states. This is called as Bohr's quantization rule.
source : http://www.upscale.utoronto.ca/GeneralInterest/Harrison/BohrModel/BohrModel.html
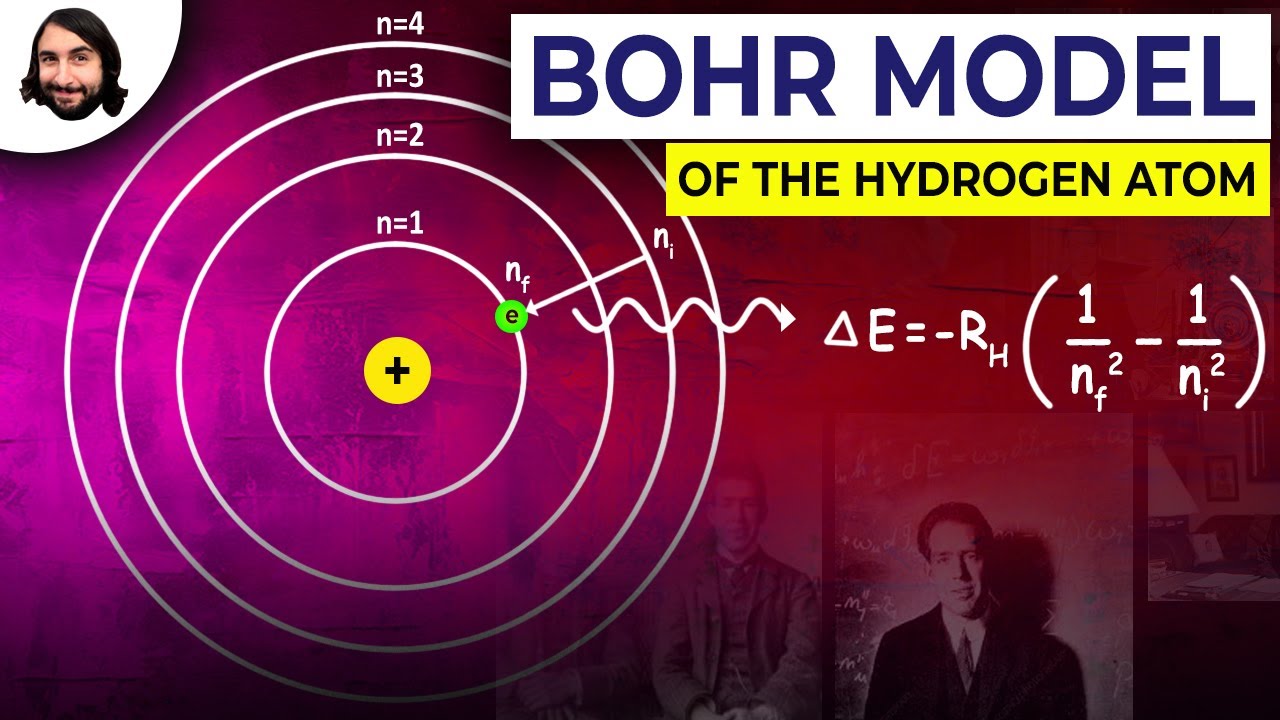
(b) The energy of the atom has a definite value in a stationary orbit. The electron can jump from one stationary orbit to another. If it jumps from an orbit of higher energy E2 to an orbit of lower energy E1, it emits a photon. The energy of the photon is E2-E1.The wavelength of the emitted radiation is given by the Einstein - Planck equation.
E2-E1= hν = hc/λ
The electron can also absorb energy from some source and jump from a lower energy level to a higher energy level as shown in the following figure.
source : http://www.tannerm.com/bohratom.htm
The figure above shows the various ways of how an electron can reach ground level after being excited to the third energy level n=3.The total energy that the electron emits as photon is hv30=hv32+hv10=hv32+hv20=hv32+hv32+hv21+hv10.
Energy of a Hydrogen Atom
The above postulates can be used to calculate allowed energies of the atom for different allowed orbits of the electron. The theory developed should be applicable to hydrogen atoms and ions having just one electron. Thus, within the Bohr atom framework, it is valid for He+, Li++, Be3+ etc. Let us consider the case of an ion with the charge of nucleus being Ze and an electron moving with a constant speed v along a circle of radius r with the center at the nucleus. The force acting on the electron is that due to Coulomb attraction and is equal to
F = Ze2/4πε0r2
The acceleration of the electron is towards the center and has a magnitude v2/r. If m is the mass of the electron, from Newton's law we obtain
Ze2/4πε0r2 = mv2/r
Using Bohr's angular momentum quantization rule for the value n, the Principal quantum number, we obtain both the velocity v, and the radius r as:
v = Ze2/2e0hnr = ε0h2n2/πmZe2 ...(i)
We see that the allowed radii are proportional to n2. For each value of n, we have an allowed orbit. For n=1, we have the first orbit (smallest radius) , for n=2, we have the second orbit and so on.
The kinetic energy of the electron in the nth orbit is
K.E = mv2/2 = mZ2e4/8ε02h2n2 ...(ii)
The potential energy of the atom is
P.E = -Ze2/4πε0r = -mZ2e4/4ε02h2n2...(iii)
Bohr Atommodell
We have taken the potential energy to be zero when the nucleus and the electron are widely separated. The total energy of the atom is
E = K.E+P.E = -mZ2e4/8ε02h2n2 ...(iv)
Equations (i) to (iv) give various parameters of the atom when the electron is in the nth orbit .The atom is also said to be in the nth energy statein this case. In deriving the expression for the total energy E, we have considered the kinetic energy of the electron and the potential energy of the electron-nucleus pair. It is assumed that of the acceleration of the nucleus is negligible on account of its large mass (i.e. that the reduced mass of the system is the same as the electron mass- this can be corrected easily later).
Radii of different Orbits
From equation (i) the radius of the smallest circle allowed to the electron is (n=1)
r1 = ε0h2/πmZe2
For hydrogen atom Z=1 and substituting the values of other constants we get r1=0.0529..nm.This length is called the Bohr radiusand is a convenient unit for measuring lengths in atomic physics. It is generally denoted by the symbol a0.The second allowed radius is 4a0 and the third allowed radius is 9a0 and so on. In general, the radius of the nth orbit is
Bohr Atom Energy Levels
rn = n2a0.
For a hydrogen-like ion with Z protons in the nucleus
rn = n2a0/Z. ...(v)
Ground and Excited states
From equation (iv) the total energy of the atom in the state n=1 is
E1 = -mZ2e2/8ε02h2
For hydrogen atom Z=1and substituting the values of the constants E1=-13.6 eV. This is the energy when the electron revolves in the smallest allowed orbit r=a0 i.e. the one with radius around 0.053nm. We also see from equation (iv) that energy of an electron is proportional to 1/n2.Thus ,
En = E1/n2 = -13.6/n2 (eV) ...(vi)
The energy in the state n=2 is E2=E1/4=-3.4 eV. In the state n=3 it is E1/9 = -1.5 eV etc. The lowest energy corresponds to the smallest circle. Note that the energy is negative and hence a larger magnitude means lower energy. The zero of energy corresponds to the state where the electron and the nucleus are widely separated. The state of atom with the lowest energy is called is ground state. The states with higher energies are called excited states. Thus the energy of a hydrogen atom in the ground state is -13.6 eV and in the first excited state = -3.4eV.
Limitations of Bohr's Model
Bohr's atomic model was ultimately not successful. It defied all attempts at improvement over the ten-year period following the original publication, and all 'obvious' improvements lead nowhere; the only 'success' was with the hydrogen atom and similar atoms i.e. those with one electron like He+, Li++ etc. Bohr's atomic model attributes a planetary motion to electrons which means that electrons move around the nucleus in defined circular orbits. This is not the modern view. The electron distribution around the nucleus of an atom is described by a probability distribution, giving rise to 'electron clouds' rather than discrete circular orbits. What survives into the new Quantum Theory is the need for single-valued wave functions, and the fact that, for hydrogen-like atoms, Bohr's model identified various dimensional parameters correctly; the rest is history.
'Every word I utter is to be understood not as an affirmation but as a question.'
-Neils Bohr
Return to Background Information home page
Related Pages :
http://www.colorado.edu/physics/2000/quantumzone/ - Contains applets for spectral lines of elements.
http://www.dauger.com/orbitals - This web page has 3D animations of orbitals.
http://www.phys.virginia.edu/classes/252/Bohr_Atom/Bohr_Atom.html - More about history of Bohr's atom.

http://www.colorado.edu/physics/2000/quantumzone/bohr.html - Interactive applets to understand Bohr's atom.
References used:
H.C.Verma - Concepts of Physics-2
Stephen Gasiorowicz - Quantum Physics
Latest version of this document: 24 May 2004Bohr created the first model that accounted for the emission of specific frequencies of light from an excited hydrogen atom.
The Bohr model is derived using three statements.
(1) The energy of the electron in a hydrogen atom is the sum of the KE and the PE. The magnitude of the kinetic energy is determined by the movement of the electron. The potential energy results from the attraction between the electron and the proton.
(1) |
Z = # of protons, e = charge of an electron, r = radius)
(2) The force that keeps the electron in its orbitis generated by the attraction of the electron for the nucleus.
So,
(2) |
(3) Since experimentation reveals that the energy of an electron in a hydrogen atom must be quantized, Bohr postulated that the angular momentum (mvr) of the electron must be quantized.
(3) |
So, rearranging equation (3) gives
Substituting for r in equation (2) and solving for v gives
Substituting for v and r in equation (1) gives
Substituting for v again gives
This simplifies to
The Bohr equation is in agreement with the Rhydberg equation, which is written below.
What Did Bohr Say About Atoms
RH is the Rydberg constant, 2.18 x 10-18 J.
What is the energy and wavelength of the photon released when an electron moves from quantum level 3 to quantum level 2?
Bohr Atom
Bohr Atom
(the negative sign tells us that energy is released)
The energy that is released is released as a photon. From the photon's point of view the photon is gaining energy (the photon is being created).
Ephoton = huEphoton = h(c/l)
3.03 x 10-19 J = (6.626 x 10-34 J s)(2.9979 x 108 m s-1)/l
l = 6.56 x 10-7 m
l = 656 nm (red)
Bohr Atomic Models Worksheet Answers


Although the Bohr atom correctly accounts for hydrogen line spectrum, the model can not be extended to other atoms. Treating an electron as a particle fails to produce a model which can describe all the elements.
